
|
Back Lesson 21
|
Home Cover Page
|
Top Lesson 22
|
Next Lesson 23
|
Lesson Twenty Two: The Nitrogen Cycle
Materials Needed. Diagram of the nitrogen cycle, legumes with root nodules (optional), small potted plants, nitrogen fertilizer, reference materials on the nitrogen cycle.
Teaching methodology: - Group work, Lecture, Experiment as project work and Report writing.
Introduce the topic by posing focus questions
Engage
The teacher is required to provide each group with the diagram of the nitrogen cycle. Using these diagrams, resources and textbooks, students will trace the flow of nitrogen through the ecosystem.
Divide students into small groups and assess their understanding of the key concepts by letting them to read the introduction about the nitrogen cycle.
What is nitrogen fixation?
Nitrogen is a component of many organic molecules. It forms an essential part of amino acids (which make up proteins) and DNA. Nitrogen is essential for all living cells. Most of the nitrogen on Earth is found in the atmosphere. It comprises approximately 78% of our atmosphere where it exists as nitrogen gas. Nitrogen can also be found in a variety of forms in plants, animals, soils, ocean, and other reservoirs in the environment. All plants and animals need nitrogen to make amino acids, proteins, and DNA, but the nitrogen in the atmosphere is not in a form that they can use. This gas must first be converted into a usable form during a process known as nitrogen fixation. Only specialized bacteria in soil and certain types of algae in water can fix nitrogen. Lightening can also result in some nitrogen fixation.
Plants get the nitrogen that they need from the soil or water in which they live. This nitrogen is usually in the form of inorganic nitrate (NO3-). Nitrate is easily dissolved in water and often leaches out of the soil. Animals get the nitrogen that they need by consuming plants or other animals which contain nitrogen within organic molecules. When organisms die, their bodies decompose bringing the nitrogen into soil or into the oceans. As these dead organisms decompose, nitrogen is converted into inorganic forms such as ammonium salts (NH4+) by a process known as mineralization. These ammonium salts are absorbed by the clay in the soil and are chemically altered by bacteria into nitrite (NO2-) and then nitrate (NO3-). The different path in which nitrogen may follow as it cycles throughout the earth is known as the nitrogen cycle.
It enters the food chain by means of nitrogen-fixing bacteria and algae in the soil. This nitrogen which has been 'fixed' is now available for plants to absorb. These types of bacteria form a symbiotic relationship with legumes these types of plants are very useful because the nitrogen fixation enriches the soil and acts as a 'natural' fertilizer. The nitrogen-fixing bacteria form nitrates out of the atmospheric nitrogen which can be taken up and dissolved in soil water by the roots of plants. Then, the nitrates are incorporated by the plants to form proteins, which can then be spread through the food chain. When organisms excrete wastes, nitrogen is released into the environment. Also, whenever an organism dies, decomposers break down the corpse into nitrogen in the form of ammonia. This nitrogen can then be used again by nitrifying bacteria to fix nitrogen for the plants. Nitrogen is essential to living things for the production of proteins, and DNA which are used to pass on the hereditary information from parent to offspring. Life cannot occur without these compounds. Even though the atmosphere is about 78% nitrogen gas, plants and animals are unable to use nitrogen gas directly as a source of nitrogen to make organic nitrogen compounds.
In order for plants to make use of nitrogen it must first be changed into nitrates in the form of nitrate ions (NO3-) dissolved in soil water. There are two pathways by which nitrate ions can be produced:
Pathway 1: Nitrogen Fixation by Lightning
The electrical energy of lightening causes nitrogen gas (N2) to react with oxygen (O2) in the atmosphere to produce nitrate ions (NO3-) which reach the soil dissolved in precipitation. Recent research suggests the amount of nitrates produced by lightening may be as high as 50%.
Pathway 2: Nitrogen Fixation by Bacteria
Bacteria in the soil can change nitrogen gas (N2) into ammonia (NH3) which then dissolves in water to form ammonium ions (NH4+). Several species of bacteria are involved. Some of these bacteria live freely in soil, while other species form a relationship (mutualism) and live in the nodules on roots of legumes such as clover or pea plants.

Fig. 22.1: Root Nodules of Legumes
Nitrification is a bacterial process in which ammonium ions are converted into nitrate ions. The ammonium ions created by the process of bacterial nitrogen fixation (described above) are changed first into nitrites (NO2-) by the action of bacteria. Then the nitrites are converted into nitrates (NO3-) by a different group of bacteria which live in the root nodules of leguminous plants.
Assimilation: is the process by which plants use the nitrate ions (NO3-) to make amino acids, proteins, and DNA only the plants and some bacteria are able to carry out the process of assimilation. All other living organisms receive their nitrogen compounds from the foods they eat. Plants therefore assimilate the proteins and then pass these compounds along the food web.
During this step, nitrites are changed into nitrogen gas (N2) which returns to the atmosphere. This step is essentially the reverse of nitrogen fixation and nitrification. The bacteria which carry out denitrification are anaerobic. The term anaerobic means, "in the absence of oxygen." This means that the bacteria can only carry out the process of denitrification in the absence of oxygen, such as when the soil is compacted.
This action can be reduced by the action of earthworms and other organisms that burrow into the soil allowing oxygen to permeate the soil environment. Except for the process of assimilation, the recycling of nitrogen is essentially a series of bacterial processes which are essential for life here on Earth.
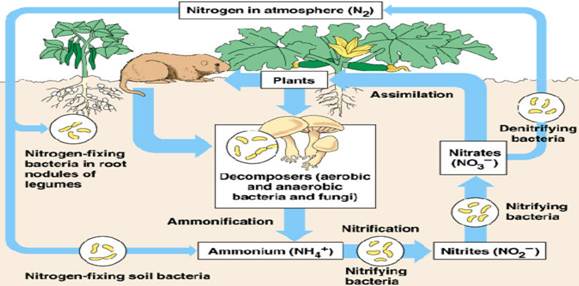
Fig. 22.2: the Nitrogen Cycle
As a class project, grow small potted plants with and without nitrogen fertilizer to determine experimentally the effect of nitrogen on the growth of plants. This project should be started at the beginning of the academic year. Students in groups should strictly observe and record any observable changes using chart. Finally, the students are required to prepare a written report to the class during the actual allotted period for the nitrogen cycle. If possible, exhibit a legume with nodules on its roots and explain its relationship to the nitrogen cycle.